Background
The quality of reporting in a research paper—its completeness, clarity, and accuracy—is crucial for its use in further research or clinical practice. Poor reporting practices are best documented in medical research1 , but other scientific disciplines are not immune to them.2 Articles that do not provide enough information about study design, methods, or findings prove difficult to index correctly in bibliographic databases, retrieve in targeted searches, assess for relevance and the presence of biases, incorporate into systematic reviews, or use in clinical practice.3 Reporting shortcomings might not be immediately obvious to readers who only read an article quickly, but they become a major obstacle for a thorough and determined reader, such as a systematic reviewer or clinical-guideline developer. Producing a well-written article that is suitable for different user groups requires a delicate act of balancing what is necessary information for inclusion and what is not.
Reporting guidelines have been developed to help authors in writing up their studies and to help editors and peer reviewers in assessing the completeness of research reporting. This article briefly introduces the EQUATOR Network and its online resources for good reporting of health research, describes key reporting guidelines, and discusses the practical aspects of implementing the use of reporting guidelines in journals.
EQUATOR Network
The EQUATOR (Enhancing the Quality and Transparency of Health Research) Network was launched in 2008 with the aim of improving the reliability and usability of health-research literature by facilitating transparent and complete reporting of research studies. It is the first coordinated effort to do that on a global scale. The work of EQUATOR supports and advances the work of the individual groups that develop reporting guidelines, such as the CONSORT Statement for reporting randomized trials. The most important output of the EQUATOR Network is a unique comprehensive online collection of reporting guidelines and other resources that support responsible publication of research (the EQUATOR Library for health research reporting www.equator-network.org/library/). Figure 1 shows the Web page outlining the current content of the library. EQUATOR supports the use of the resources through dedicated toolkits4 and through various education and training events that target editors, peer reviewers, and authors.5
Reporting Guidelines
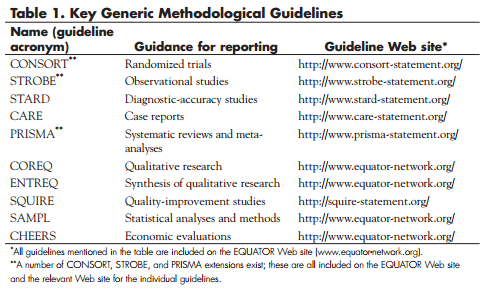
Reporting guidelines provide structured advice on what information needs to be included in a research article as a minimum to allow readers to assess study methods, relevance, and validity of presented findings. They focus on the scientific content of an article and thus complement journals’ instructions for authors. The EQUATOR Network’s freely available online library lists more than 200 reporting guidelines. Some are generic methodological guidelines for different types of study designs (such as randomized trials, systematic reviews, and observational studies) that should always be followed in reporting these types of studies. The primary focus of the guidelines is on the description of study methods and corresponding advice on reporting study findings. The content of each of the guidelines has been carefully considered by multidisciplinary groups of relevant experts and stakeholders, and there is a strong rationale for each item of requested information. Table 1 lists the key generic methodological guidelines. Most of the guidelines listed in the EQUATOR library are more specific, providing guidance relevant to a particular medical field (for example, reporting immunotherapy trials) or a particular aspect of research or of a research report (for example, reporting of statistical analyses, adverse events, and specific procedures). These guidelines ideally are used in conjunction with the generic method-focused guidelines.
Implementation of Reporting Guidelines in Journals
The Recommendations for the Conduct, Reporting, Editing, and Publication of Scholarly Work in Medical Journals— formerly the Uniform Requirements for Biomedical Manuscripts, the fundamental guidelines developed by the International Committee of Medical Journal Editors— encourage the use of reporting guidelines and EQUATOR resources.6
The EQUATOR Web site features a dedicated toolkit section for journal editors and peer reviewers that has been developed to support editors in setting up more rigorous policies on health-research reporting and to improve the relevant parts of their instructions for authors and peer reviewers.7 The EQUATOR editors’ toolkit also provides practical suggestions and experiences from other journals on how to select and use appropriate reporting guidelines in editorial and peer review.
Relevant method-focused guidelines (listed in Table 1) should be the first reporting guidelines considered for implementation, and journal contributors should be clearly instructed to adhere to them. Specialty journals might also consider investigating whether any specific guidelines focus on their clinical field. Many such guidelines were developed by professional societies and provide useful specific guidance that facilitates better comparison of results between studies.
Ensuring adherence to relevant guidelines can be difficult for editors. Invaluable help can be provided by asking reviewers to use the relevant checklists as part of their assessment and to highlight reporting shortcomings in a systematic way.
Improving the Quality of the Health-research
Literature Research and its publication constitute a global enterprise. Most journals are not bound by geography; they are international in scope but focus on specific clinical fields. The focused and yet global nature of research publishing has profound implications for improving reporting quality and the reliability of research literature. Journal editors can play a fundamental role in that process. Possible solutions can include a “vertical” or “horizontal” approach. For example, a vertical approach can involve measures that ensure transparency and completeness of research by visibly linking the individual elements of the whole research process: study registration, availability of protocols and ethical-approval documents, journal publication, and the public availability of findings. The horizontal approach can involve a collaborative effort by editors in a particular clinical field to develop common editorial policies and harmonize their instructions on research reporting. That might lead to improvements in the reporting of research across the field and might prevent authors from resubmitting manuscripts of substandard reporting quality to different journals in the field until they find a publishing outlet. Harmonizing publication requirements throughout a field can also minimize the possible adverse effect on the number of submissions that a particular journal may receive if it is the only one that introduces more stringent criteria. The whole field can then be seen as leading the way in promoting research-publication excellence. Cardiovascular journals are a good example of such collaboration. The European Society of Cardiology National Cardiovascular Journals and the HEART (Heart Editors Action Round Table) group issued various consensus statements to promote editorial excellence, which also include recommendations to encourage adherence to the CONSORT Statement for reporting randomized trials.9
Similar efforts to improve the quality of research reporting and thus increase the reliability and usability of available evidence can be seen outside human medicine.10–13
Concluding Remarks
Adherence to reporting guidelines is a simple but effective way to improve the completeness and usability of research reports. However, reporting guidelines are only guidelines. They provide a minimum set of reporting recommendations, and their use has to be driven by common sense. Only the researchers who conducted a study know what factors could have influenced their study findings, and they need to report all the key study details accurately and honestly. The role of editors and peer reviewers is to remind researchers of that duty and, when possible, ensure that they have fulfilled it. The EQUATOR Network resources will be helpful tools in that process.
Acknowledgment
The author thanks Professor Doug Altman and Mrs Shona Kirtley, of the Centre for Statistics in Medicine, Oxford, for their valuable comments.
Declaration of interest
Iveta Simera’s salary is paid by EQUATOR Network program grants. The EQUATOR Network is supported by the UK National Institute for Health Research, the UK Medical Research Council, the Scottish Chief Scientist Office, and the Pan American Health Organisation. None of those funders influenced the content of this paper.
References
- Simera I, Kirtley S, Altman DG. Reporting clinical research: Guidance to encourage accurate and transparent research reporting. Maturitas 2012;72:84–87.
- Sargeant JM, Elgie R, Valcour J, Saint-Onge J, Thompson A, Marcynuk P, Snedeker K. Methodological quality and completeness of reporting in clinical trials conducted in livestock species. Preventive Veterinary Medicine 2009;91:107–115.
- Simera I. Reporting guidelines: a tool to increase completeness, transparency, and value of health research published in your journal In: Smart P, Maisonneuve H and Polderman A. (eds) Science Editors’ Handbook. European Association of Science Editors, 2013, pp. 27–30.
- EQUATOR Toolkits. www.equator-network.org/toolkits/ (accessed 12 December 2013).
- EQUATOR Education and Training events. www.equator-network.org/category/events/all-past-events/ (accessed 12 December 2013).
- Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. www.icmje.org/ (accessed 12 December 2013).
- EQUATOR Editors Toolkit. www.equatornetwork.org/toolkits/editors/ (accessed 12 December 2013).
- Hirst A, Altman DG. Are peer reviewers encouraged to use reporting guidelines? A survey of 116 health research journals. PLoS One 2012;7:e35621.
- Alfonso F, Ambrosio G, Pinto FJ, Ector H, Vardas P, Kulakowski P, and Timmis A on behalf of the Editors’ Network ESC Task Force. European Society of Cardiology National Cardiovascular Journals: the ‘Editors’ Network’. European Heart Journal 2010;31:26–28.
- Newman M, Elbourne D. Improving the Usability of Educational Research: Guidelines for the REPOrting of Primary Empirical Research Studies in Education (The REPOSE Guidelines). Evaluation and Research in Education 2004;18:201–2012.
- Jedlitschka A, Pfahl D. Reporting Guidelines for Controlled Experiments in Software Engineering, Proc. Int’l Symp. Empirical Software Eng. 2005;95–104.
- More SJ. Improving the quality of reporting in veterinary journals: How far do we need to go with reporting guidelines? The Veterinary Journal 2010;184:249–250.
- Editorial. Enhancing reproducibility. Nature Methods 2013;10:367.
IVETA SIMERA is head of program development at EQUATOR Network, Centre for Statistics in Medicine, Oxford, United Kingdom.
